In 1984, radiation worker Stanley Watras set off an alarm that detected radiation exposure. A quick examination revealed Watras was not physically carrying any radiation source; the explanation was much more insidious: His body had absorbed large amounts of radon gas from his house’s basement.
We tend to believe radiation is relatively rare and dangerous, but actually it’s all around us. Recall the Periodic Table that hung on the wall of your high school science class. All those elements have isotopes, or variations that contain different numbers of neutrons in their nuclei. Some of these isotopes are stable, but others are unstable: they’re radioactive and release high-energy waves or particles. In fact, elements like uranium exist only in an unstable form.
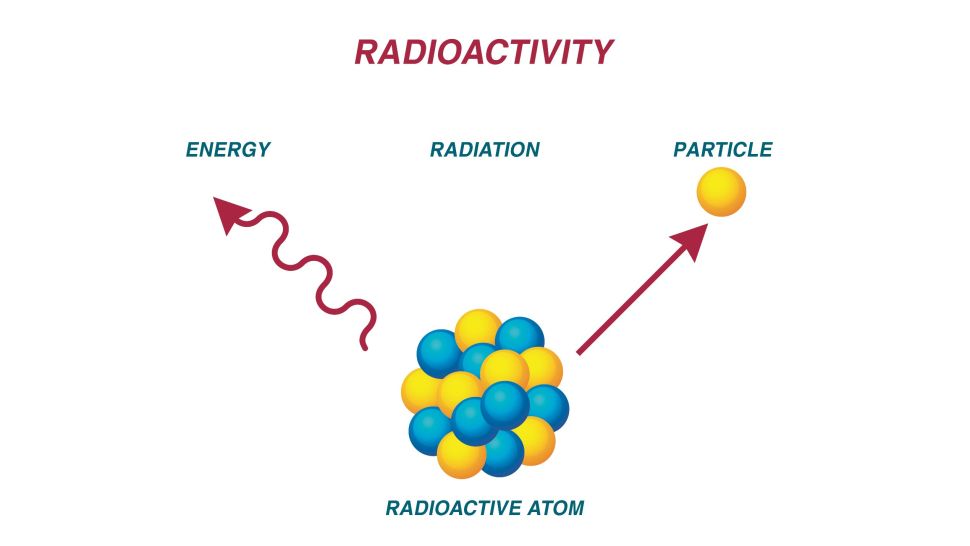
We get affected because many of these isotopes and radioactive elements occur naturally in the environment and can be absorbed by plants and water. So we probably ingest very small amounts of isotopes every time we eat food or drink. As Mr. Watras learned, unventilated basements, especially those with large deposits of granite, are another source. We also absorb radiation when we fly, like my trip to Europe earlier this month. And all those atmospheric tests of nuclear weapons in the 1950s and 1960s didn’t help, either.
Despite the ubiquity of radiation in our environment, this is a matter to be taken seriously — for example, high amounts of radon gas increase the risk of lung cancer. So radiation is another reason to be environmentally aware of the risks around us.
For more information, see “How Radioactive Is The Human Body?” by Jacklin Kwan (https://www.livescience.com/radiation-human-body?). The illustration came from that article.
